In what is considered a medical breakthrough, scientists from Linköping University in Sweden have restored vision to people with impaired eyesight. This includes those who were previously blind.
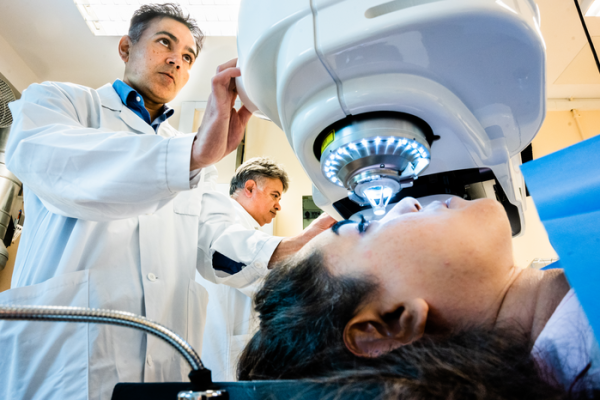
A team of surgeons in Iran and India conducted a successful pilot trial of 20 people who were either blind or close to losing their sight from advanced keratoconus. They used bioengineered corneas from pig skin, as described in Nature Biotechnology. This innovation could help restore sight to people in countries where human cornea transplants are in short supply, and for a lower price.
Unlike human corneas, which must be transplanted within two weeks, the bioengineered implants can be stored for up to two years, which could help with shipping them to those who need them the most.
A scientific breakthrough in restoring eyesight
Corneal blindness is a big problem: around 12.7 million people are estimated to be affected by the condition. Every year, cases are rising at a rate of around a million. Iran, India, China, and various countries in Africa have particularly high levels of corneal blindness, and specifically keratoconus.
The cornea implant is made from collagen protein extracted from pig skin. Pig skin has a similar structure to human skin. Purified collagen molecules were processed to ensure that no animal tissues or biological components remained.
 The team, from Linköping University in Sweden, then stabilized the loose molecules into a hydrogel scaffold . The scaffold is designed to mimic the human cornea, which is robust enough to be implanted into an eye.
The team, from Linköping University in Sweden, then stabilized the loose molecules into a hydrogel scaffold . The scaffold is designed to mimic the human cornea, which is robust enough to be implanted into an eye.
The study
Surgeons in Iran and India conducted a pilot trial of 20 people who were either blind or close to losing their eyesight from advanced keratoconus. This disease thins the cornea, the outermost transparent layer of the eye, and prevents the eye from focusing properly.
The implant restored the cornea’s thickness and curvature. All 14 of the participants who had been blind before the operation had their vision restored. Three of them actually achieved perfect 20/20 vision.
Safer cornea transplants
While human cornea transplants in patients with keratoconus are traditionally sewn into sutures, the team experimented with a new surgical method that’s simpler and potentially safer.
They used a laser to make an incision in the middle of the existing cornea before inserting the implant, which helped the wound heal more quickly and created little to no inflammation afterwards. Consequently, the patients only needed to use immunosuppressant eye drops for eight weeks, while recipients of traditional transplants usually needed to take immunosuppressants for at least a year.
One unexpected bonus was that the implant changed the shape of the cornea enough for its recipients to wear contact lenses for the best possible vision, even though they had been previously unable to tolerate them.
The cornea helps focus light rays on the retina at the back of the eye and protects the eye from dirt and germs. When damaged by infection or injury, it can prevent light from reaching the retina, making it difficult to see.
Affordable eyesight
Scientists say that as pig skin is a by-product of the food industry, using this bioengineered implant should cost a fraction as much as transplanting a human donor cornea.
Neil Lagali, a professor at the Department of Biomedical and Clinical Sciences at Linköping University, one of the researchers behind the study commented, “It will be affordable, even to people in low-income countries. There’s a much bigger cost saving compared to the way traditional corneal transplantation is being done today.”
Approval to take this eyesight device to market

Dr. Mehrdad Rafat Phd
The safety and effectiveness of bioengineered implants have been at the core of the team’s work.
Mehrdad Rafat, PhD, is adjunct associate professor at LiU’s department of biomedical engineering and founder and CEO of LinkoCare Life Sciences, which manufactures the bioengineered corneas used in the study. He explained further.
“We’ve made significant efforts to ensure that our invention will be widely available and affordable by all and not just by the wealthy. That’s why this technology can be used in all parts of the world.”
The team is hoping to run a larger clinical trial of at least 100 patients in Europe and the US. They are also working on the regulatory process required for the US Food and Drug Administration to approve the device for the market.

![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)










