South Africa, 23 March 2022: An exciting new clinical trial on a plant-based PHELA to treat COVID-19 has been announced. The Department of Pharmacology at the University of the Free State (UFS in South Africa) and FARMOVS have teamed up to conduct the trial. It will be the first South African Health Products Regulatory Authority (SAHPRA)-approved multi-center controlled clinical trial of a plant-based product, PHELA, on mild to moderate COVID-19 patients.
A COVID-19 treatment
Prof Motlalepula Matsabisa, professor and Director of Pharmacology at UFS, said the trial would start in early April 2022. Each patient will be on treatment for 28 days.
“The main purpose of the clinical trial is to confirm that the product can treat COVID-19 and be registered as a medication for this indication. We believe the medication works as an immune modulator to modulate the cytokine storm due to COVID-19 and also restores and normalises the patient’s immune system. We plan to have 250 patients who suffer from mild to moderate COVID-19,” explains Prof Matsabisa.
A collaborative clinical trial
This pivotal study is based on the modification of the World Health Organisation (WHO) Master protocol for clinical trials.
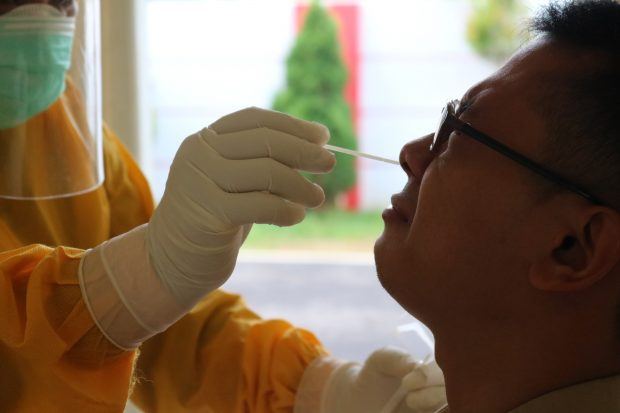
Photo by Mufid Majnun on Unsplash
What is PHELA?
Prof Matsabisa, is deputy president of the South African Society for Basic and Clinical Pharmacology Society (SASBCP). He says the development of PHELA has been under stringent scientific scrutiny for its safety in both preclinical and clinical research.
The efficacy of PHELA as both an immune modulator and an anti-SARS-COV-2 has been proven in vitro and in vivo. The product showed reproducible results as conducted by three independent research institutions and a science council.
Using medicinal plants in the clinical trials
The Department of Pharmacology and FARMOVS are collaborating on a number of studies to advance clinical research on African Traditional Medicines (ATM).
On the use of PHELA, Prof Matsabisa explains:
“PHELA is a herbal product made of four medicinal plants. Traditionally, PHELA has been claimed for use for a historical disease called muyaga, but has recently been scientifically tested and found effective as an immune modulator, benefiting persons with a compromised immune system.
“The PHELA plants are found in most provinces of South Africa, and we have cultivated them to control their growth to produce quality raw materials.”
“The SAHPRA-approved clinical trial will be conducted in the Eastern Cape, Northern Cape, and Gauteng. The clinical trial will be conducted by a complement of medical staff and clinicians with vast experience of many active years of clinical trials.”
PHELA is an immune reconstitution product
“The study, we believe, is a benchmark for all future traditional medicine clinical trial protocols and studies. The study is expected to start immediately after the product batch manufacturing of the study product, PHELA, is completed, and this will be within a month’s time.“
A lot of good scientific preclinical safety and efficacy research has gone into the development of the study product for it to reach this stage.
“The efficacy studies have shown convincingly that PHELA is an immune reconstitution product and does have an effect in killing the SARS-COV-2 virus and most of its variants. PHELA efficacy, therefore, needs to be confirmed through randomised controlled multi-centre clinical trials in COVID-19 patients,” Prof Matsabisa says.
Medicinal plants have previously been used to eradicate life-threatening viruses
Although medicinal plants have been used to combat previous pandemics such as the Spanish flu, avian influenza, and others, we still believe rigorous control and efficacy thereof is still to be supported by scientific research and development, says Prof Matsabisa.
Prof Matsabisa is also the current chairperson of the World Health Organisation’s (WHO) Regional Expert Advisory Committee on Traditional Medicines (REACT).
He adds: “We have better technologies and resources now. This is why we should take the next step in research to promote consumer safety and offer them effective alternatives. We do science to aid in building the herbal industry and developing sustained consumer confidence in traditional medicines.“

Africa should lead the way to a healthier future for all
“My vision is for Africa to share our valuable resources with the world by developing and distributing world-class medicinal solutions. We should develop and strengthen the pharmaceutical local production of well-researched, quality, safe, and efficacious African traditional medicines as commercial products.
We are more than capable of doing so and now is the time to do it. Numerous discussions have taken place where other African countries will join South Africa in conducting multi-centre studies in clinical trials for traditional medicines.
“We need to develop or create, based on this current collaborative work with partners like FARMOVS. As well as health centers with a strong focus on African medicines, health products, and healing, but in a very strong collaborative initiative with other health systems”, concludes Prof Matsabisa.
FARMOVS is a wholly-owned clinical research company on the UFS Bloemfontein campus. For this clinical trial to take place they had to implement collaborative initiatives between UFS and FARMOVS on clinical research, training, and other research projects.
About the clinic lead
 Prof Motlalepula Matsabisa is a professor and Director of Pharmacology at the University of the Free State (UFS). He was recently awarded a Visiting Professorship at the Beijing University of Chinese Medicine (BUCM) in Beijing, China.
Prof Motlalepula Matsabisa is a professor and Director of Pharmacology at the University of the Free State (UFS). He was recently awarded a Visiting Professorship at the Beijing University of Chinese Medicine (BUCM) in Beijing, China.
He was also recommended by Naledi Pandor, Minister of International Relations and Cooperation. This enabled him to be part of the India, Brazil and South Africa (IBSA) working group in traditional medicine.
Prof Matsabisa has participated in the national department of health technical committee on traditional medicines where he has been appointed by the Minister of Health, Dr Joe Phaahla.
Additional references:
Evaluation of traditional medicines. Identification of PHELA using different chromatographic techniques
Makhotso Lekhooa 1 , Andrew Walubo, Jan J B Du Plessis, Motlalepula C Matsabisa, Duduzile Molefe
https://pubmed.ncbi.nlm.nih.gov/23983353/
A case study of Phela: Research Gate: https://www.researchgate.net/publication/344767142_A_case_study_of_Phela_an_African_herbal_medicine_on_evidence_based_research


![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)










