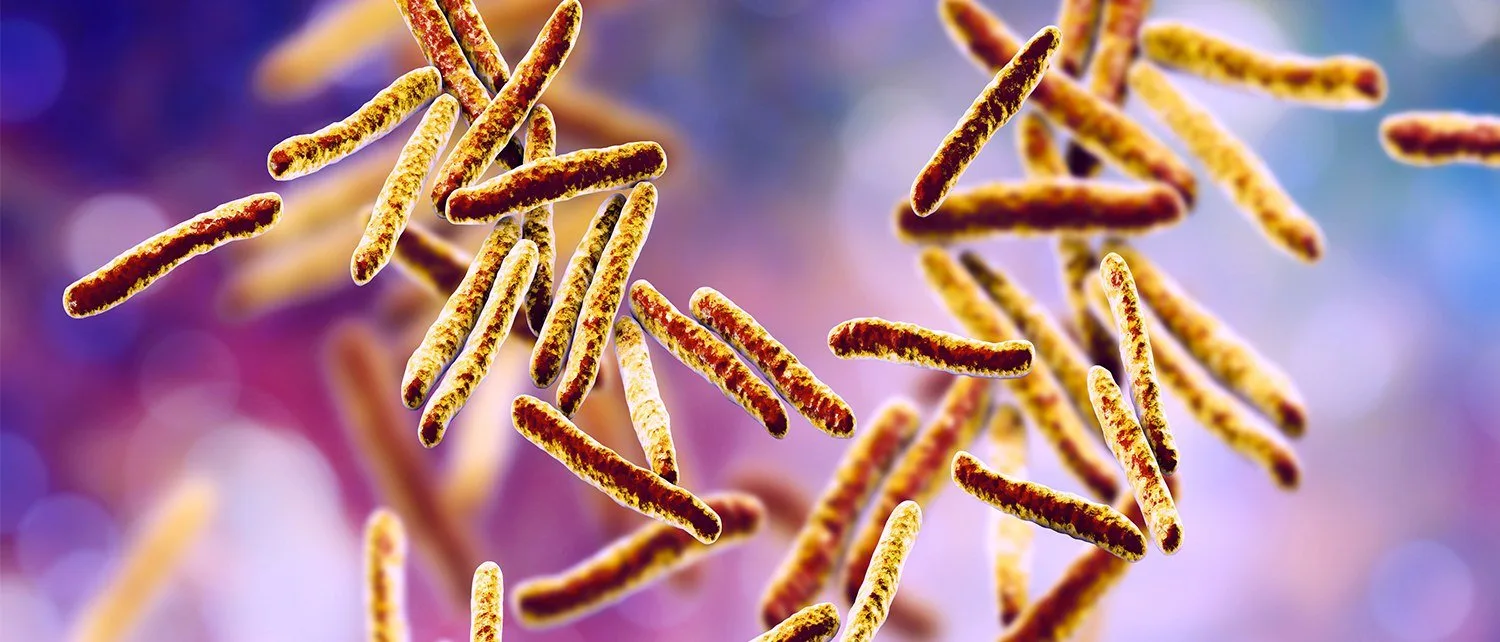
Until quite recently, people suffering from highly drug-resistant tuberculosis had poor treatment options and a poor prognosis. However, a new medicine for people with the worst cases of TB is now giving hope where almost none existed. Developed by scientists at the New York–based non-profit TB Alliance, the new pill is called pretomanid. The U.S. Food and Drug Administration (FDA) has approved its use in conjunction with two other antibiotics — bedaquiline and linezolid. Tests indicate that the three-drug regimen has a 90 percent cure rate for patients suffering from the most lethal forms of tuberculosis.
New Tuberculosis Treatment Approved by the FDA
Pretomanid is only the third anti-TB drug approved by the FDA in more than 40 years. The new treatment could soon be available worldwide, as the World Health Organization typically follows the FDA’s lead in approving new medicines. The World Health Organization said that 10 million people fell ill from TB in 2018 and the disease claimed 1.6 million lives.
Tuberculosis has now surpassed AIDS as the world’s leading infectious cause of death. While only a small fraction of patients with tuberculosis contract the deadliest strain of the disease, very few survive it. But the new drug regimen, says the FDA, cures most of these patients within months.
Through effective diagnosis and treatment, 53 million lives were saved between 2000 and 2016, and the number of TB-related deaths continues to drop by around 2 percent each year, according to the latest figures from the World Health Organization.
Drug-Resistant TB is a Growing Concern
The World Health Organization says highly drug-resistant TB has been reported in more than 120 countries. And there are some 558,000 new cases reported in 2017. This includes a smaller subset of patients with extensively drug-resistant TB, the deadliest strain targeted by the new drug regimen. Until now, the prescribed treatment was lengthy and complicated, requiring as many as eight kinds of shots and pills. Patients often die well before completing their treatment cycles.
Despite significant progress by scientists each year, tuberculosis is still one of the top causes of death worldwide.
The disease is the leading cause of death among those living with HIV, who are 20 to 30 times more likely to succumb to airborne disease than those without HIV. The United States — through the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) — works to combat this by screening people living with HIV for tuberculosis. In 2017 they trained 250,000 health care professionals globally.
The World Health Organization has set an ambitious goal for the global community: to reduce TB deaths by 95 percent between 2015 and 2035 and to cut new cases by 90 percent during the same time period. “At WHO, we will be moving with urgency as a united force,” said Dr. Tereza Kasaeva, director of the World Health Organization’s Global Tuberculosis Programme.
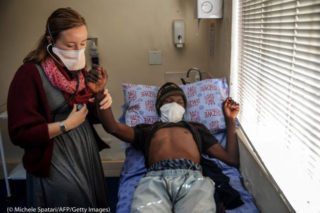
A physician (left) examines a tuberculosis patient at the Sizwe Tropical Diseases Hospital in Johannesburg on August 5. (© Michele Spatari/AFP/Getty Images)
TB Alliance is working with the WHO
The first United Nations General Assembly High-Level Meeting on TB will occur in November in New York. It follows last November’s inaugural Global Ministerial Conference to End TB, when leaders and activists from 120 countries gathered in Moscow and promised a new global commitment to do more to combat the disease.
The TB Alliance said it will work with the World Health Organization to speed adoption of the all-oral, three-drug combo in countries where highly drug-resistant TB is endemic, potentially helping more than 75,000 patients each year.
Contributed by Lauren Monsen with additional notes.

![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)