The National Health Service (NHS), like all health services, won’t just take on a new instrument. And even with their rigorous process, they sometimes still approve the wrong one. Gloves that rip easily, cannulas that do not go in as easily, bed pans that leak. Longevity Live Paid Content.
Still, the process at least ensures things are safe, most of the time. This process ensures that only the most effective and safe equipment is introduced into the healthcare system.
Below, we’ll explore what that process is and how it benefits patient outcomes.
Identifying the Need for New Medical Equipment
The NHS begins with identifying a specific need or gap in the current medical practice. And there’s often a gap.
It could be keeping up with evolving medical procedures, outdated tools, or the need to enhance patient safety and outcomes. They’ll conduct a thorough assessment to understand how the new instrument can improve clinical practices. They’ll also compare it to existing solutions. After all, sometimes change isn’t the best thing.
Research and Development
The next step involves research and development (R&D). This stage is critical in designing and developing a new medical instrument. For example, this phase often requires collaboration between medical professionals, engineers, and manufacturers to create a tool that is both innovative and practical.
Clinical Trials and Evaluation
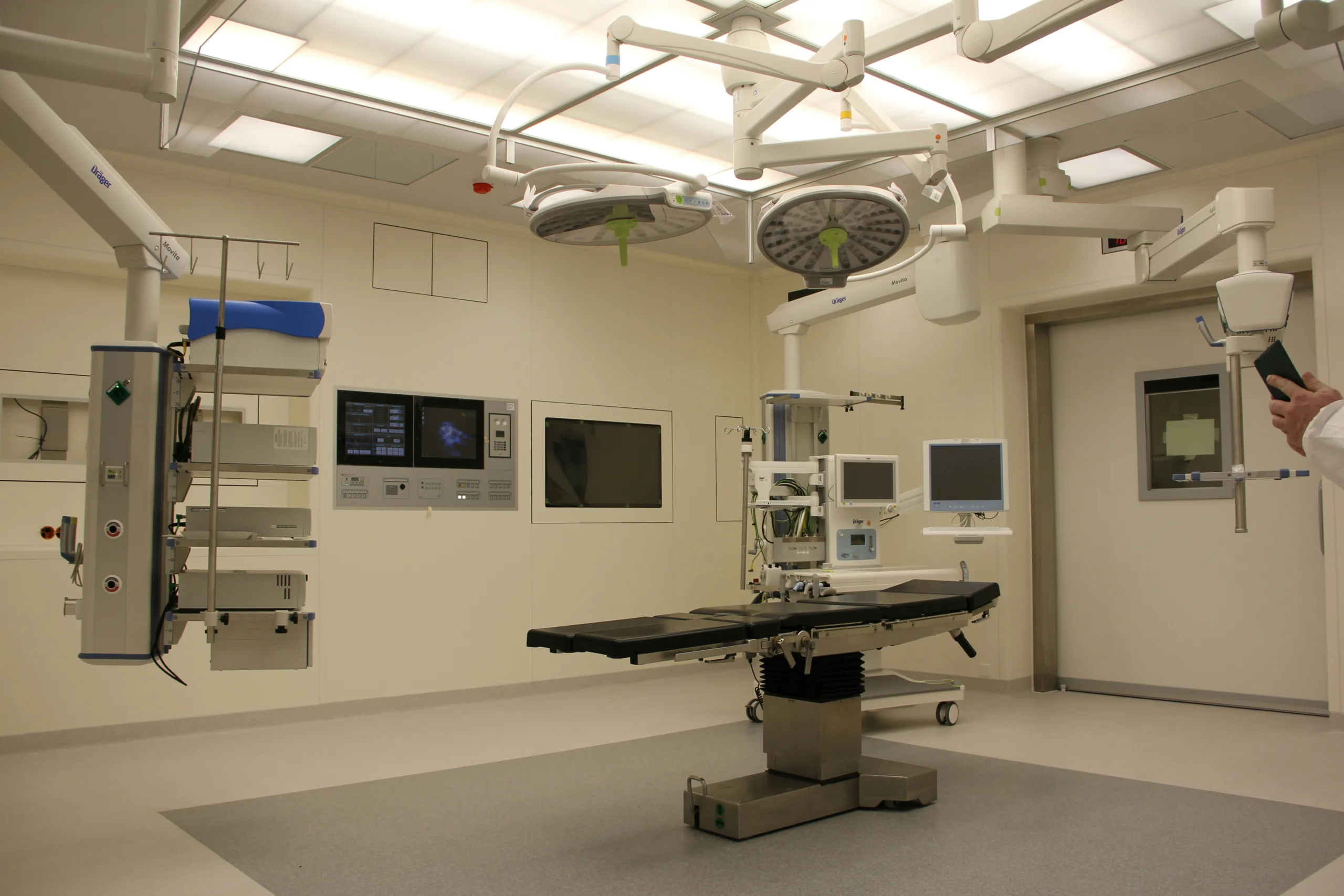
The medical instrument undergoes rigorous clinical trials and evaluations. It has to work safely. The NHS can’t afford claims of malpractice because of faulty equipment.
It’ll also test efficiency and performance in a controlled environment. You can’t go straight for patient trials. And some of them don’t even need patient trials.
Clinical trials are essential to gather evidence on the instrument’s benefits and potential risks. The data collected will support the effectiveness of the instrument.
Regulatory Approval and Compliance
Then there’s regulatory approval. That involves compliance with the Medicines and Healthcare Products Regulatory Agency (MHRA) standards. The approval process ensures that the instrument meets all the required safety, quality, and efficacy standards. Again, sometimes it fails.
There also must be compliance with legal and ethical guidelines. It’s crucial to safeguard patient welfare and uphold medical standards.
Spread the Message and Adoption
The next step is the spread and adoption of the innovation. That involves raising awareness among potential adopters within the NHS. That could be healthcare professionals and administrators.
Effective dissemination and diffusion strategies are employed to promote the new instrument.
But they don’t always work. Influential individuals within medical and surgical fields can play a significant role in advocating the adoption of instruments like the surgery retractor by June Medical, for example, or new airway masks, showcasing their benefits and practical applications in clinical settings.
Sustaining the Innovation
The innovation must be sustained. There’s ongoing monitoring and evaluation of the instrument’s performance, gathering feedback from medical professionals, and making necessary adjustments or improvements. Sometimes, equipment doesn’t always last long before the brand changes.
And there’s a need for continuous education and training for healthcare providers. If they don’t know how to use it properly, it won’t work as well.
It’s a long process. Sometimes, it can take years for new instrument adoption. Most of it comes down to money, or lack of it.
Still, it’s designed to be effective and safe equipment integrated into patient care. It’s these instruments that are improving patient care and outcomes.

![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)










