A South African biotech company has received approval from the European Union (EU). The company manufactures disinfectants with 99.987% efficacy against SARS-CoV-2 (Covid-19). At the end of a very long, and expensive five years of testing and following a laborious, strict application process, Biodx has finally achieved EU registration for their b bioactive™ (DECONT-X™) disinfectant technology.
Biodx is the first SA biotech firm to receive the stringent EU Biocidal Registration for a biocidal product. Biocidal products typically destroy harmful organisms. They are also made using active ingredients that may be a natural substance or a chemical substance. Biodx has made their active ingredient, b bioactive™, using natural citrus extract. This makes it safe for humans, hands, and the environment.
In a series called The Business of Health, hosted on the Nielsen Network, Longevity Live spoke to Biodx CEO Burt Rodrigues.
What was the process of getting approval from the European Union?
“The International Chemical Regulatory Framework especially bioscience is very strict. As you can imagine depending on the status of your country’s scientific adherence and compliance to international standards and ability to perform, research and interpret data accurately, come up with a product that is directly related to the challenge and the difficulty of registering a bio-sign.”

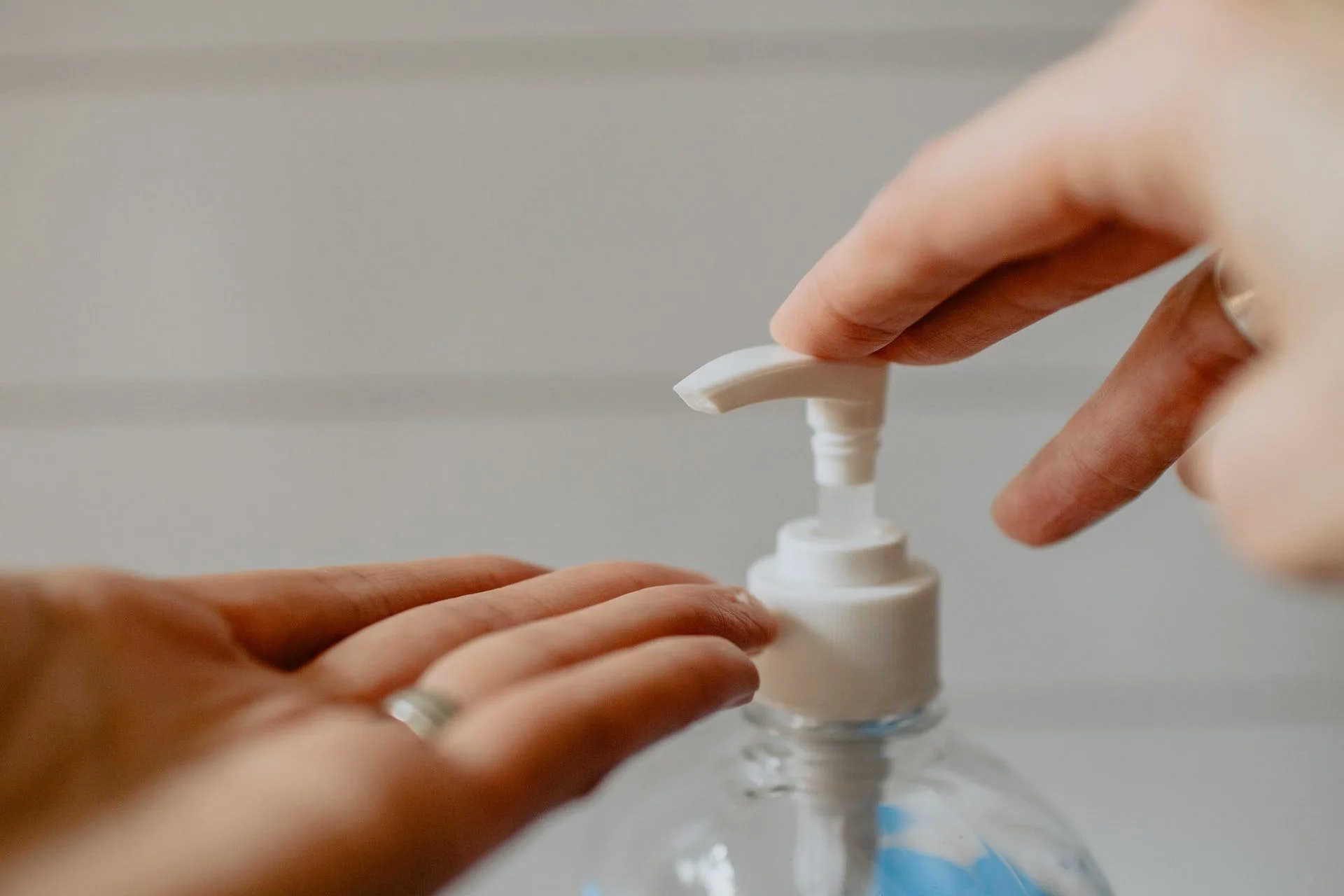
Photo by Trust “Tru” Katsande on Unsplash
“Registering a bio-sign in South Africa has its own complexity because we have the South African Bureau Standards. Second, you have to go MRCS which is a compulsory standard for disinfectants, and you must register. It’s not left for your choice. Products can kill lives. Bioscience is meant to kill, however, in controlled environmental conditions. That in itself, in South Africa, may be a four-to-five-year process and cost you R200 000 – R500 000. In Europe, because the competencies in the sciences are much higher, they demand so much more. As such, all the test work needs to be redone in Europe”.
The product has been specifically formulated as an active ingredient for the following:
- hand sanitisers
- surface cleaning disinfectants
- surface disinfectant wipes
- industrial preservation products
- food preservation products
- agri-business disinfection,
- preservation and anti-microbial fogging for interior inanimate environments
It can be used directly or in formulations across many environments. These include;
- healthcare
- science and technology
- hospitality
- retail
- commercial
- industrial
- agriculture
- domestic
Biodx is on a journey to reduce society’s dependence on synthetic chemicals. Harnessing the power of biotechnology, breaking boundaries, crossing new frontiers, and helping to evolve the future of disinfection. All towards enabling a better world.
What is the impact of continuous use of disinfectors on humans?
“The overuse of any chemical compound on the skin will create sensitisation over a period of time. Many of the products that you are putting on your hands, especially the hand sanitisers, are usually tested 10 times, use per day by the manufacturer of the component, whether it’s an alcohol-based hand sanitizer or water-based. It may contain an active ingredient that is lower in percentage. However, the manufacturer will test this product for you to use a maximum of 10 times a day. Those are the safety claims that you’re provided with as a consumer”.
Watch The Video
The video interview contains the full dialogue of this interview, and you can watch it below.


![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)