In the rapidly advancing world of gene therapy, the journey from concept to cure is intricate and multifaceted. Central to this journey is the production of viral vectors, the delivery vehicles that carry therapeutic genes into human cells. This is where Contract Manufacturing Organizations (CMOs) specializing in viral vectors play an indispensable role. A viral vector CMO not only brings expertise in the complex bioprocessing required, but also navigates the regulatory landscape, ensuring that these critical components are produced to the highest standards. Longevity Live Paid Content.
Why Viral Vectors?
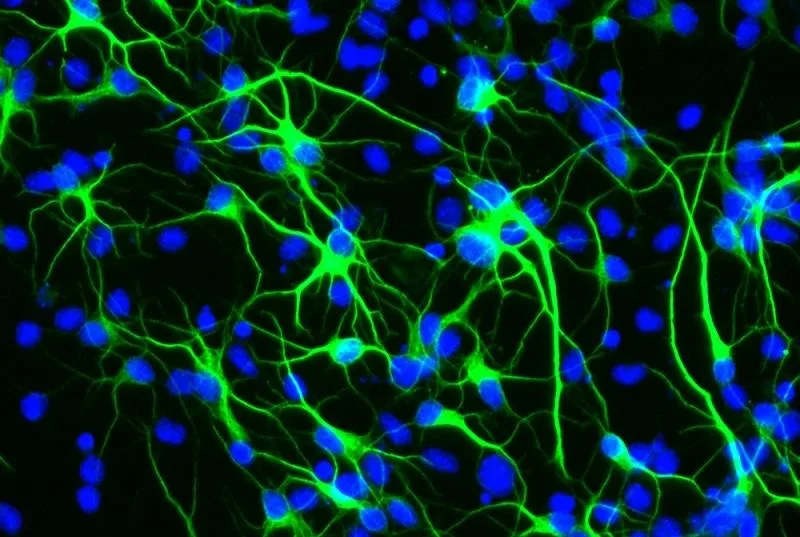
Viral vectors are at the heart of gene therapy, offering a means to correct or compensate for genes that cause disease. Their design and production, however, require precision, as the vectors must effectively deliver therapeutic genes without causing harm to the patient. This balance of safety and efficacy is achieved through cutting-edge biotechnological processes, making the role of CMOs critical in the gene therapy supply chain.
The Expertise of CMOs
CMOs in the gene therapy space are not just manufacturers; they are partners in innovation. With capabilities ranging from early-stage development through to commercial-scale production, a viral vector CMO works closely with therapy developers to bring treatments from the lab bench to the bedside.
Their expertise covers a broad spectrum, including vector design, process development, manufacturing under Good Manufacturing Practice (GMP) conditions, and rigorous quality control.
Scaling Up Challenges
One of the significant challenges in gene therapy is scaling up viral vector production without compromising quality. As treatments progress through clinical trials and into commercialization, the demand for viral vectors increases exponentially. CMOs are at the forefront of addressing this challenge, employing scalable processes and innovative bioreactor technologies to meet the growing needs of the gene therapy market.
Navigating Regulatory Waters
The path to commercializing gene therapy is fraught with regulatory hurdles. Here, the experience of CMOs is invaluable. They ensure compliance with global regulatory standards by navigating the complexities of the FDA, EMA, and other regulatory bodies. Their quality assurance processes are rigorous, ensuring that every batch of viral vectors meets the strictest safety and efficacy standards.
The Future of Viral Vector Production
As the demand for gene therapies continues to grow, so too does the need for efficient, scalable viral vector production. Innovations in bioprocessing, such as the use of suspension cell cultures and transient transfection techniques, are on the rise. These advancements promise to increase yields, reduce costs, and accelerate the production timeline, ultimately bringing life-saving therapies to patients faster.
Impact of CMO Partnerships
The partnership between gene therapy developers and CMOs is a cornerstone of the industry’s success. These collaborations allow therapy developers to leverage the CMO’s expertise and focus on their core competencies, such as clinical research and patient care. As reported by Reuters, the strategic importance of CMOs in the pharmaceutical industry has never been higher, underscored by the increasing investment in this sector.
Looking Ahead
The landscape of gene therapy is evolving, with new treatments on the horizon that promise to tackle previously untreatable conditions. As we look to the future, the role of CMOs in this ecosystem will continue to expand. Their ability to innovate, scale, and ensure the quality of viral vectors will be paramount in realizing the full potential of gene therapy.
Engaging in this dynamic field requires not just scientific expertise, but a vision for the future. As highlighted by The Guardian, gene therapy represents one of the most promising frontiers in medicine, with the potential to change countless lives. The contributions of CMOs to this field are invaluable. It’s driving forward the development of gene therapies that could one day cure a wide array of genetic diseases.
Your Thoughts
The journey of developing and producing viral vectors for gene therapy is complex and fraught with challenges. Yet the promise it holds for treating genetic disorders is unparalleled.
What do you think are the biggest hurdles in viral vector production? How can collaborations with CMOs overcome these challenges? Share your thoughts and join the discussion on the future of gene therapy and the vital role of CMOs in shaping this future.
Who is the author?

Helen Bell
Helen Bell is a medical writer who primarily focuses on recent developments in the pharmaceutical industry. In addition, you may find her writing for your favorite self-development blogs.

![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)










