AUSTRALIA, 26th September 2023. Non-profit organisation Mind Medicine Australia has issued a media statement affirming Australia’s decision to provide restricted rescheduling of MDMA and Psilocybin for therapeutic purposes.
On 3rd February 2023, the Therapeutic Goods Administration (TGA) announced the restricted rescheduling of MDMA and Psilocybin as Schedule 8 Controlled Medicines when used as part of therapy for patients with post-traumatic stress disorder (MDMA) or treatment-resistant depression (psilocybin). As part of the decision, the TGA acknowledged the current lack of treatment options for patients suffering from these mental illnesses, which can cause immense suffering.
The TGA’s decision was based on a wealth of evidence presented by Mind Medicine Australia in its rescheduling applications, as well as on the TGA’s own investigations and feedback from over thirteen thousand public submissions. The submissions were 98% in favor of rescheduling and included thousands of positive submissions lodged by clinicians and researchers.
Two major international trial results support the use of MDMA and Psilocybin under controlled conditions
It is therefore highly relevant that two major trials, which have just published their results in leading medical journals, provide further support for using these medicines in the controlled conditions envisaged by the TGA.
The trial sponsors in each case were not-for-profit organizations focused on the alleviation of suffering, and both had successfully obtained Breakthrough Therapy Status from the FDA in the United States to fast-track the assessment of these medicines because of their enormous potential.
The results of the two trials are summarized as follows:
1. The MAPS Second Stage Phase 3 Trial Report on MDMA – assisted therapy
The MAPS Second Stage Phase 3 Trial Report “MDMA-assisted therapy for moderate to severe PTSD: a randomized placebo-controlled phase 3 trial” was published in Nature Medicine on 14 September 2023. This multi-site trial confirmed the impressive results of the MAPS first stage Phase 3 trial and the MAPS Phase 2 trials. In particular, following the treatment with MDMA-assisted therapy:
• 86.2% of participants in the MDMA group noted improved symptoms, compared to 69% in the placebo group.
• 71.2% of participants in the MDMA group also no longer met the criteria for PTSD, versus 47.6% in the placebo group.
• Participants in both groups were drawn from ethnically diverse backgrounds, indicating the broader generalizability of the treatment.
• Covariate analyses demonstrated similar responses to treatment regardless of disease severity, risk of hazardous alcohol or substance use disorder, severe adverse childhood experiences, or dissociative subtype PTSD.
• Reported side effects of the treatment, including muscle tightness and nausea, were in line with expectations. There were no serious treatment-emergent adverse effects or challenges such as suicidal ideation.
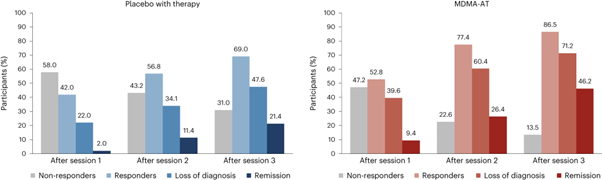
Table 1 reproduced from Fig 3 of the Nature Medicine Article showing the treatment response and remission rates of participants in the MDMA assisted therapy and placebo plus therapy groups. Sample sizes for both of the MAPS Phase 3 trials were developed with guidance from the FDA to ensure adequate and rigorous testing of outcomes during the trial period.
MAPS has indicated that it will be lodging an application later this year for MDMA to be registered as a medicine in the United States when used as part of therapy. The TGA’s rescheduling decision is more limited and only applies to the use of MDMA as an unregistered medicine.
2. Usona Institute Phase 2 Trial
The Usona Institute Phase 2 Trial: “Single-Dose Psilocybin Treatment for Major Depressive Disorder” published in JAMA on 31 August 2023 is a large multi-site, randomized, multi-blinded, placebo-controlled trial. The study was designed to evaluate the magnitude, timing, and durability of antidepressant effects and the safety of a single dose of psilocybin combined with therapy in patients with major depressive disorders. The trial followed a successful Compass Pathways trial using a single dose of psilocybin for treatment-resistant depression, published in The New England Journal of Medicine on 3 November 2022.
Research conclusions
The research conclusions were that the psilocybin administered with psychological support was associated with a rapid and sustained antidepressant effect, measured as a change in depressive symptom scores, compared with the active placebo group. Psilocybin was generally well tolerated, and there were no serious treatment-emergent adverse effects in the psilocybin group. Importantly, the treatment with psilocybin didn’t cause any emotional blunting, which can be a side effect of antidepressant use for some patients.
According to the Chair of Mind Medicine Australia, Mr Peter Hunt AM: “These results further demonstrate why the TGA’s rescheduling decision is so important for patients suffering from PTSD or from treatment resistant Depression. These are debilitating illnesses which can cause immense suffering. The challenge now is to make sure that government controls don’t unreasonably restrict patient access.”
Executive Director of Mind Medicine Australia, Tania de Jong AM, explains: “We now will be able to collate Real World Evidence about the application of these therapies in clinical practice through the registry specifically set up for this purpose by the Australian National University. This is a world first.
The controlled clinical application of these therapies to help patients suffering from debilitating conditions doesn’t preclude ongoing clinical trials, which Mind Medicine Australia is also strongly supporting.”
About Mind Medicine, Australia
Mind Medicine Australia is Australia’s leading not-for-profit organization working on the use of medicinal psilocybin and MDMA-assisted therapies to treat a range of mental illnesses.


![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)










