Generic medication saves Americans billions every year. In fact, it has been shown that they have saved U.S. consumers over $1 trillion in the past decades. While these lower-priced variations are great for your wallet, just how great are they for your overall health and well-being?
What Is A Generic Drug?
A generic drug is a pharmaceutical drug that contains the same chemical substances as a drug originally protected by chemical patents. They are mainly designed to act as equal substitutes to their original counterpart. Basically, this is a drug that has been created to be the same as an already marketed brand-name drug. These similarities are in dosage form, safety, strength, administration, quality, performance characteristics, and intended use.
However, over the years, these drugs have come under scrutiny for various reasons. With the difference in pricing and ingredients, people are questioning their safety, quality, and efficiency. While generic drugs are FDA-approved, the process of approval doesn’t really seem to guarantee safety.
Key Differences In Drug Approval By The FDA
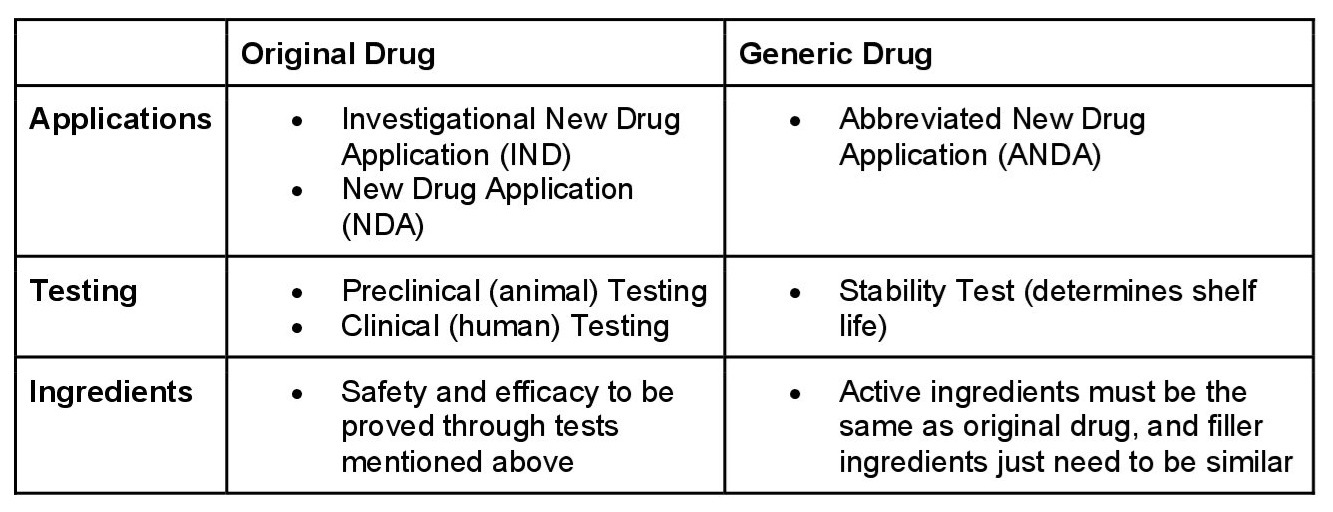
Approval Process
FDA gives patent and exclusive protection to pharmaceutical manufacturers. This allows them to profit from their research and innovation for several years. The patent applies well before clinical trials have begun testing the drug’s safety and efficacy. During this time, no generics can be produced to compete with the brand. However, once this patent expires, generics can be produced through a shortened FDA approval process.
In order to acquire a grant for this approval, the generic drug must be “bioequivalent” to its brand-name counterpart. Bioequivalent means that it works the same way, and provides the same benefits. So, chemically, the two must be similar, with manufacturers allowing 20% variation in the active ingredient from that ingredient formula.
No Tests Conducted
Pharmaceutical companies need to have a chemical recipe that is almost identical to the original drug. However, they don’t have to show that the two versions are therapeutically equivalent. Basically, they do not need to conduct tests to establish the drug’s safety, or ability to work the same way as the original drug. As long as the ingredients appear similar, the FDA doesn’t require them to be tested. According to their website, “the ANDA process does not require the drug applicant to repeat costly animal and clinical studies on ingredients or dosage forms already approved for safety and effectiveness“.
What Makes Up A Generic Drug?
The build of a generic drug needs to contain the same active ingredients, and in the same amount, as the original. Active ingredients are a substance in a product that has an effect on the body. It can either affect one part of your body (such as your skin), or the entire body. These chemicals provide a biologically active effect in the cure, treatment, or prevention of disease. While the other ingredients in the pills can be different, there is a limit.
The FDA allows for a 15% difference, either more or less, in blood absorption for a generic compared to the original drug. One analysis of 2000 studies found that the average difference between generic drugs, and original drugs, was about 3.5%.
Why Are They More Affordable?
Generic drugs can cost as much as 80% less than an original drug. Part of the reasoning is that creating a new drug is expensive. Manufacturers must cover costs for the launch of the new drug, which includes research and large-scale drug testing. Unlike original drugs, generic medicines do not have to repeat animal and clinical studies to establish safety and effectiveness. This could pose a problem for our overall health and safety.
Is Safety Really Guaranteed Then?
A Canadian study aimed to show the difference between generic and brand-name drugs. In this study, they specifically looked at blood pressure drugs. They raised whether patients experienced more side effects from generic drugs when compared to the original one.
The researchers looked at the number of emergency room visits and hospitalizations in 136,777 individuals aged 66 and over. They examined data 24 months before and 12 months after the intake of generic versions of blood pressure drugs. It was found that after implementing the generic drug, the rates of hospitalization went up (by 8% for losartan, 12% for valsartan, and 14% for candesartan).
What Causes These Adverse Reactions?
If you experience an adverse reaction to generic medication, it may not be due to the active ingredient in the drug, but the “inactive ingredients” in it. A recent study examined the molecular interaction of “inactive” filler ingredients. By systematically screening thousands of these ingredients previously thought to be dormant, they found that many of them are biologically active and cause unintended physiological effects.
Inactive Ingredients
While the most important part of the medicine is the active ingredient, inactive/filler ingredients can make up to 99% of a drug. These ingredients serve various purposes, from bulking up the tiny active molecules, so they can be turned into a pill, to enhancing their therapeutic potential. An example of this is caffeine. Generic pain-relieving medication often contains this filler ingredient.
Studies have shown that “a dose of caffeine equivalent to a mug of coffee added to a standard dose of painkillers provides better pain relief.” However, with caffeine sensitivity affecting so many people, prescription painkillers that are caffeine free are now being manufactured.
If your original drug contains no caffeine (due to your caffeine sensitivity), taking the generic version (which would typically contain caffeine) can cause adverse effects, including anxiety, chest pains, headaches, trembling, and jitters.
Research suggests that many of these filler ingredients can be harmful.
“While the doses may be low, we don’t know what the threshold is for individuals to react in the majority of instances,” says gastroenterologist Giovanni Traverso from MIT, Brigham and Women’s Hospital.
Possible Contaminants?
Inactive ingredients aren’t the only things we have to be aware of. According to a UBC study, contaminants in generic medications used to treat conditions such as heart disease, diabetes, and heartburn, amongst others, may cause damage to DNA, affect basic cell functions, and even increase a person’s risk of cancer. One of these contaminants is N-nitrosamine.
First noted as an impurity of heart medication, this toxin has led to multiple medication recalls by the FDA and Health Canada. Many chronic generic medications contain it. According to Dr. Corey Nislow, “When we take these drugs, we do so knowing that they can come with side effects that are clearly described on the drug label. But what we don’t expect is that our medicine cabinet may be full of toxins that can make us sick – or kill us.”
Should We Cancel Generic Drugs?
This isn’t to say that you shouldn’t be taking generic medication. If we look at the healthcare and pharmaceutical industry, medication is quite expensive. Generic drugs are a critical part of a financially sustainable healthcare system. With the FDA sharing that generic drugs can cost 20-70% less than their original counterpart, they are a cost-effective way to obtain your prescription medication. However, what should be prioritized is speaking to your doctor.
You need to establish which active ingredients you need to be consuming, and the filler/inactive ingredients that may lead to adverse effects. This allows you to make an informed decision that is based on your individual health. Medication treats individuals differently, so weigh the drug variations against your specific needs and allergies.


![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)










