This week, the world’s largest biotech company Roche announced that the company’s cobas® HPV test has been awarded prequalification status by the World Health Organization (WHO). This prequalification expands the availability of this critical HPV screening tool. This augurs well for helping with the early detection of cervical cancer.
Why is screening for HPV so important?
Screening for Human Papillomavirus (HPV) can help identify women who are at risk of developing cervical cancer so that the disease can be treated early before invasive cancer has a chance to develop. In poorer countries, women are often diagnosed with cervical cancer at a more advanced stage, where the opportunity for a cure is low.
“The elimination of cervical cancer is within reach. Roche is committed to working with governments, non-profit organizations, and funders to help build sustainable cervical cancer elimination programs so that women, no matter where in the world they live, no longer die from this preventable disease,” said Joni Zurawinski, Advocate for Equal Access to Healthcare at Roche Diagnostics.
“Today’s action, combined with our recently-launched HPV-self sampling solution, further expands access to HPV screening in countries with limited healthcare resources.”
The World Health Organisation’s Strategy to eliminate cervical cancer
The WHO strategy for global elimination of cervical cancer lists the following three target goals to reach by 20302:
- 90% of girls should be fully vaccinated with HPV vaccine by 15 years of age;
- 70% of women should be screened using a high-performance test by age 35, and again by age 45;
- 90% of those identified with cervical disease should receive appropriate treatment.
The cobas® HPV test is already part of the Roche Global Access Program, which aims to improve access to cost-effective resources, implement scale-up programs, and contribute to the elimination of diseases in the regions with the greatest need.
WHO prequalification helps expand that access and provides healthcare professionals with greater confidence that their clinical decisions will be based on accurate, reliable results.
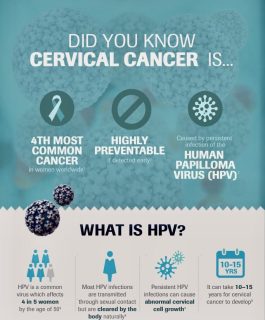
Infographic courtesy of Africa Cancer Foundation
About the Global Access Program
In 2014, Roche first launched its Global Access Program to support the UNAIDS 2020 targets to address the HIV/AIDS epidemic. Since then, the program was expanded to include solutions for other high-burden diseases such as Tuberculosis, Hepatitis B and C, and cervical cancer. Most recently, in response to the COVID-19 pandemic, the SARS-CoV-2 test was included into the program.
About the cobas HPV test
The cobas® HPV test is indicated for use for routine cervical cancer screening as per professional medical guidelines, including HPV primary screening, co-testing (or adjunctive screen) with cytology, and for triage of women with abnormal cytology, to assess the risk for cervical precancer and cancer. The cobas® HPV test detects the high-risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
In June 2022, Roche further improved access for women when it launched an HPV self-sampling solution in countries accepting the CE mark. The solution enables a patient to privately and confidently collect her own sample following instruction from a healthcare worker. The clinically-validated vaginal sample is then analysed with the Roche cobas® HPV test on a Roche molecular instrument.
Cervical cancer screening using the cobas® HPV test is clinically validated in large, FDA registrational trials for use on cobas® Systems, and the assay individually identifies the presence of the DNA of HPV genotypes 16 and 18 – the two genotypes responsible for about 70 percent of all cervical cancers – and reporting the 12 other high-risk HPV types as a combined result, all in one test and from one patient sample.
References and additional information
More information about the cobas® HPV tests is available at diagnostics.roche.com/cervicalcancer.
The fully automated cobas® 6800/8800 Systems offer the fastest time to results, providing up to 96 results in about three hours and 384 results for the cobas® 6800 System and 1,056 results for the cobas® 8800 System in an eight hour shift. Learn more: diagnostics.roche.com.
About Roche
Roche is the world’s largest biotech company, with truly differentiated medicines in oncology, immunology, infectious diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics, tissue-based cancer diagnostics, and a frontrunner in diabetes management. The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in 2020 employed more than 100,000 people worldwide. In 2020,

![women [longevity live]](https://longevitylive.com/wp-content/uploads/2020/01/photo-of-women-walking-down-the-street-1116984-100x100.jpg)










